Contact
Director: Eric Rosenberg, M.D. (617)724-7519
Associate Director: John Branda, M.D. (617)-726-3611
Associate Director: Virginia Pierce, M.D. (617)726-3612
Assistant Director: Sarah Turbett, M.D. (617)724-7648
Lab Hours
Microbiology Specimen Receiving
24 hours for accessioning and processing of specimens.
Bacteriology, Molecular Microbiology, Mycology, Mycobacteriology, Parasitology, Serology, and Virology
Monday-Friday: 7:00 AM – 4:00 PM
For information or special test requests call (617)726-3613 during the above hours. For after-hour emergency assistance call the Clinical Pathology Resident for Microbiology (beeper #2-1826).
Description
The Microbiology Laboratories are divided into Bacteriology, Susceptibility Testing, Mycology, Mycobacteriology, Parasitology, Virology, Serology, and Molecular Diagnostics. Specimens are accepted for accessioning and processing for all divisions 24 hours/day.
Specimen Collection
Specimens submitted to the Microbiology Laboratories must be labeled with patient’s name and unit number and according to MGH Specimen Labeling Policy. The specimen should be bagged according to Infection Control Standard Precaution protocols. Do not place patient labels on the specimen transport bags.
If a requisition is used, the requisition should be firmly attached to the outside of the specimen transport bag and be filled in with the following information:
- Patient Name
- Patient Unit Number
- Patient Location (i.e., in-house, clinic or physician to whom report is to be sent if private patient).
- Telephone extension of ordering care unit or physician.
- Type or source (anatomic site) of specimen.
- Type of culture requested (i.e. routine, fungal, TB, viral, etc.)
- Ordering Physician
- Diagnosis and/or specific organism sought.
- Date and time of collection.
- Specific organism sought.
Specimen Delivery
Because they may contain viable micro-organisms, specimens should be delivered to the laboratory as soon as possible after collection. Specimens are accepted for all divisions 24 hours per day/7 days per week.
Special Requirements
Ambulatory patients requiring specimens for parasite examination should be directed to the Ambulatory Blood Lab in the Wang Ambulatory Care Center (WACC), 2nd Level for specimen collection instructions and assistance. Fresh stool specimens for O&P arriving in laboratory after 4 p.m. will be preserved for processing the next working day.
Respiratory specimens from cystic fibrosis patients (including post-transplant patients) must be labeled “CF” to receive appropriate specimen processing and workup Please make sure to specify “CF Patient” in the Special Request field in the Epic order.
Criteria for Refusal of Specimens
For the purpose of providing useful diagnostic testing, the Microbiology laboratories consider it an obligation to refuse specimens under the following circumstances:
- Patient’s name and unit number are not provided.
- Name on specimen differs from that on the requisition or SQ Lab Label.
- Specimen type is not that indicated or is different than on the requisition.
- Specimen is one or more days old.
- Test requested is not available in the Microbiology Lab.
- Quantity is not sufficient.
- Quality of specimen is questionable.
- Improperly sealed or leaking container.
- Specimen is received in fixative or in improper fixative.
- Time or date of collection is not indicated.
- Excessive delay in receiving specimen for anaerobic culture.
- Excessive delay in receiving specimen for urine culture.(Inpatient specimens >4 hours. Outpatient specimens > 24 hours without refrigeration).
- Method of collection is not acceptable for anaerobic culture and viral culture.
- Stool specimens from patients hospitalized >3 days.
- Whole blood specimens for molecular diagnostics testing received >6 hours after collection.
Specimens that have been refused are held in the laboratory for 48 hours and will be processed only after consultation with the laboratory supervisory personnel by the requesting physician. Specimens which are unacceptable for any of the above reasons, which have been collected by invasive procedures, or which might necessitate recalling an outpatient for recollection will usually be processed with a notation on the report indicating why the specimen is unacceptable. Interpret results of such cultures carefully.
Results
Preliminary and final times listed in Lab Handbook for individual cultures indicate the earliest times for availability of results. Positive cultures may require additional time for identification or susceptibility testing of isolates. The lab will communicate results to the requesting area in accordance with the MGH Communication of the Critical Results Policy
Notes on Microbiology Laboratory results (December 2009)
-
- Preliminary Results – All culture results are preliminary unless marked “FINAL.” Preliminary results may be changed, modified, deleted, or augmented after further observation, incubation, or testing of cultures.
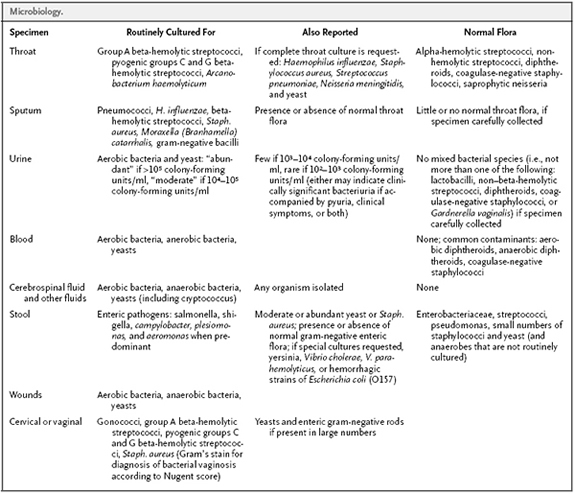
- Routine cultures and “Normal Flora” –
- Antimicrobial Susceptibility Testing – Susceptibility testing of aerobic pathogens is performed by either an automated rapid microdilution system (Vitek 2 AMS), the Kirby-Bauer disk diffusion method, a manual microdilution method, or E-test depending upon identification of the organism, reliability of the applicable methods, or specific antibiotics which may not be on the automated or manual microdilution panels. Anaerobic susceptibility testing is available after consultation with the laboratory. Results of susceptibility testing are interpreted into 3 categories (Susceptible, Intermediate, Resistant), using standardized criteria. The intermediate category for a drug indicates an equivocal result and this drug should generally not be relied on for single agent therapy except perhaps of urinary tract infections. Certain organisms are screened for beta-lactamase production before susceptibility results are reported.
